Research Paper on Adsorption Cooling with Heat from Solar Energy
Introduction and Background
The incorporation of solar energy projects into a mixed approach to alternative energy sources can be a very useful tool, particularly in geographical locales where exposure to sunlight and a temperate or arid climate holds sway. You don’t just have to set up farms in the middle of a desert and run connections to the nearest power grid for solar power to work – individual homes can be fitted with solar panels that are either dedicated to specific appliances, such as hot water heaters, or that contribute to the power needs of the entire home. Businesses can connect to larger solar arrays on the property, if not on the roof. However, the cost of solar energy arrays means that, even with government rebates, homeowners often require ten or fifteen years to earn back the cost of the solar installation from savings in utility bills. For larger-scale projects, operated by utilities and governmental entities, the costs involved, when compared to the durability of solar panels, are not controllable enough to lead to significant investment without some other benefit besides extra power to the grid. Since the 1970s, scientists have pursued alternate applications for solar power that would add to the benefits without contributing much to the costs. One of the solutions for this pursuit is adsorption cooling, which is actually a way to use energy twice, as the waste heat that comes with the storage of solar energy is used to produce a cooling effect. By giving solar energy a second way to improve energy efficiency, engineers have given public and private entities additional financial savings in the area of utilities.
As with many projects in alternative energy, interest began in the 1970s, when the world faced a crisis revolving around available oil. This included solar energy, but also included wind and geothermal power as well. Because of the prohibitive costs of massive solar arrays, other sources of efficiency were sought, and so adsorption cooling was one way to make solar energy make more sense financially, because of the additional cost savings with utilities (Wang and Oliveira). In the 1990s, a new environmental hazard arose specific to climate control, as scientists became aware that coolants released HCFCs and CFCs into the atmosphere. These two substances eat holes in the ozone layer, allowing more heat into the atmosphere and contributing the greenhouse effect, as the heat has nowhere to go. As a result, adsorption cooling became a hot topic again, as scientists and engineers began to search for other ways to eliminate as much heat as possible from different production processes (Wang and Oliveira). Current advancements in adsorption cooling make it a useful component in several different types of refrigerant appliances.
Description of the Technology or Concept
Most appliances that use adsorption cooling operate in the same basic way. With the first way, refrigerant evaporates inside the evaporation cylinder. Some sort of coolant (generally air or water) will eliminate the heat produced, leading to overall reduction in temperature (Teng, et. al.). In addition to the benefit of conserved power, by using solar energy or ambient heat, adsorption cooling also is much easier to control. It can work in environments with heat sources as low as 50 degrees Celsius and as high as 500. The liquid comes from within, and so you don’t have to pump refrigerant into the unit. The limited exposure to moisture makes corrosion much less likely. With fewer moving parts, there are fewer points of vulnerability to outside shocks (Wang and Oliveira).
However, there are some drawbacks to the adsorption cooling process. They have lower cooling overall power and specific cooling power than the same metrics in the absorption process. Different teams have worked with making the working pairs more adsorptive and by managing heat more effectively through the process.
Using the waste heat from solar energy collection, several different appliances have been designed. This paper will look at a variety of air conditioning models that use adsorption – those that have closed systems and those with open ones, and will give an evaluation of the overall effectiveness of both. In general terms, though, adsorption works according to the principles of Figure 1:
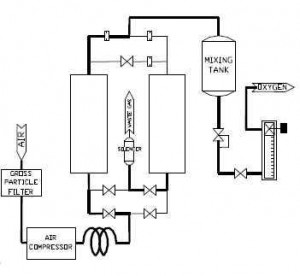
The air goes through a gross particle filter and then through the compressor, into one or more pressurized chambers. Often, these chambers will contain substances that will separate atmospheric air into its component gases, depending on the purpose of the adsorption. Some systems use adsorption to purify oxygen, for example, and will use a substance like zeolite to pull out the nitrogen as an initial step in purificatoin (Ozmotic Insiders). Because adsorption involves removing components of a substance rather than adding, though, the idea behind the process remains the same. For a look at adsorption cooling more specifically, take a look at Figure 2:
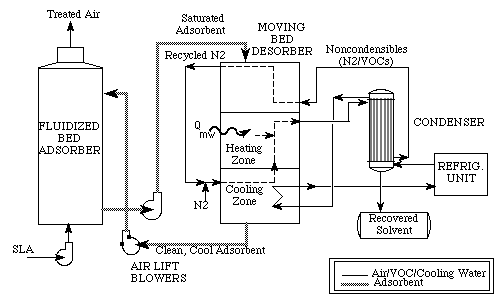
Air that is already loaded with solvent heads into the adsorber and then around through to the moving bed desorber. The refrigeration unit gets the solvent out through condensation and sends the finished air back to the desorber, for shipment through the air lift blowers, back through the fluidized bed adsorber and out, back into the air system (Price and Schmidt). Many adsorbent coolers use carbon as an adsorbent and methanol as a refrigerant (Wang and Oliveira). In order to boost cooling power, engineers have found that it is best to shorten the cycle time in which the system operates; tests on an adsorption cooling system by Wang et. al., for example, found that a cycle of thirty minutes yields cooling power of 3.84 kW, while a cycle of a full hour only yielded 3.03 kW in power.
Examples or Case Studies
A look at many of the research steps that have gone into the development of the optimal parameters for an adsorption cooler is instructive, when it comes to understanding how the refinements of the process have changed the way that the systems cool air.
Early efforts include the model put together by Grenier et. al. This system was driven by 20 square meters of solar panels and paired zeolite and water as the refrigerant pair. The purpose of this system was to cool a room with 12 cubic meters of space, designed to preserve food. As the system worked, it could refrigerate 1,000 kg of vegetables, assuming a rotation of about one seventh of the stock each day. Saha et. al. looked at a four-bed, two-stage model with a non-regenerative adsorption cooler that could use solar heat as well as waste heat from other sources, between 50 and 70 degrees Celsius. The prototype from this group had a by-product of cold water (10 degrees Celsius), and the cooling power was calculated at 3.2 kW. With the use of flat plate energy collectors, the prototype was able to produce heated water at 55 degrees Celsius, which was optimal for providing that level of cooling power consistently.
Xia et. al. devised an adsorption chiller that uses silica gel and water, with twin chambers that each have a first stage evaporator, condenser and adsorber. The tubes are joined by a recovery tube, and both chambers lead to a second stage evaporator that uses methanol. This model was used to create the cooling unit in China’s Jiangsu Province (Wang and Oliveira). The solar powered unit that heats the water consists of a water pump, storage tank and a system of evacuated tube collectors. This version uses a cooling tower to release the heat from the adsorbers and condensers, and the fan coil unit sends the cooling effect to the grain depot. Additionally, this system yields heat as well, which provides hot water to power the chiller. On Xia’s prototype, when water heated to 85 degrees Celsius is used to run the cooler, the available cooling power was almost 5 kW. When the water was 65 degrees Celsius, the cooling power was still 2.97 kW. What this means is that the solar energy collectors can provide the necessary heat to provide coolant – no outside energy is needed to heat the water that operates the cooler.
Liu et. al. came up with an adsorption cooler design that didn’t need any refrigerant valves, because it contained an internal liquid pairing of silica gel and water. Because valves are one of the moving parts that fail most frequently, this design would reduce maintenance costs dramatically, as well as make the entire unit cheaper, as fewer parts would be necessary. Using water between 75 and 90 degrees Celsius, it would be possible to regenerate the sorption bed for continual use. In Liu’s case, there were two adsorbent beds with a combined total of 52.8 kg of silica gel. The beds work out of phase, which means that one of them is always cooling the air. This prototype yielded a cooling power of 3.56 kW with an evaporation temperature of 7 degrees Celsius, heat source temperature of 85 degrees Celsius, and a heat sink temperature of 28 degrees Celsius. Some improvements that Liu’s group made after this first prototype had even fewer moving parts, so that leaks would be even less likely. In the first model, the condenser had caused refrigerant evaporation inside the machine that could lead to corrosion, and so a new condenser went into this new model that did not have that evaporation. Under testing, this newer prototype yielded 34 percent higher cooling power (Liu et. al.).
Restuccia et. al. added calcium chloride to the silica gel in their prototype, as added sorption substance. The calcium chloride, when dry, can sorb water at a rate of 0.7 kg per kg of calcium chloride, which heightened the efficiency of the adsorption process.
In addition to these closed-system adsorption cooling systems, there are open-system systems that release the water that serves as the refrigerant into the atmosphere, instead of cycling it back into the system; these systems are also referred to as desiccant systems, because they end up removing the moisture from the materials. Thoruwa, et. al. wanted to develop a desiccant system that would provide cool, dry air for grain storage buildings. The desiccant in this model was made of calcium chloride, bentonite, cement and vermiculite in a mass ratio of 1:6:1:2, and set in a flat plate collector that measured 0.9 square meters. The system would reduce the humidity of the air around the grain by 40 percent, and the dry, cool air churned out at a rate of two cubic meters per minute. The collection of solar energy during the day would regenerate the desiccant.
Ismail et. al. put together a system using silica gel as part of the working pair to cut down on the moisture in the air that was also used to remove the heat from collected grains. This model sent the air through two silica gel beds and two heat exchangers to suck the humidity out of the air. When the humidity went down during the day, the atmospheric air would regenerate the silica gel beds, and the solar energy collector would provide the heat needed to operate the chiller. The end result was a cooling down of the grain to approximately 16 degrees Celsius, which was about five degrees cooler than the ambient temperature (and significantly drier).
Henning et. al. looked at a way to use desiccant to enhance the effectiveness of an evaporative cooler. Their prototype was able to reduce the temperature of ambient air (31 degrees Celsius during testing) to 19 degrees Celsius. The system only used about 76 percent of the solar energy that it stored during the day, running the existing air through a series of dehumidifiers and through heat recovery units.
Lu and Yan developed what they called an SDERC unit (solar desiccant enhanced radiative cooling), also using silica gel as the adsorbent. By alternating the adsorption and generation phases for the array of sorption panels that lined the ceiling and side walls, Lu and Yan created a system that would always provide drier air. However, the dehumidifier worked much more effectively during the day, because the heat at work in the panels was significantly greater. Producing the same dehumidification at night was not feasible, because of the absence of solar energy.
Discussion
While open-system, or desiccant, adsorption cooling systems are now commonly in use for grain storage, and some residential evaporative coolers use adsorption technology, there are still some barriers keeping adsorption cooling from becoming a widely used technology, particularly in commercial systems. Also, while certain parts of the adsorption cooling system are cheaper, because they have fewer moving parts, as a whole, the systems are more expensive than their absorption cooling counterparts. The adsorbers also transfer heat less efficiently, which causes problems in the larger volume needs of commercial buildings. It is worth discussing whether or not heat pipes would help bridge the gap between absorbers and adsorbers in cooling systems. There are several arguments in favor of using heat pipes for this purpose.
First of all, a study at LIMSI resulted in heat transfer coefficients higher than 10 kW per square meter. This happens because, when the heat pipe’s working fluid condenses, the process produces enough heat to regenerate the adsorber. Using solar energy, heat pipes can carry materials through the refrigerant and heat transfer fluid circuits. The solar energy provides enough heat to run the chiller, because the working fluid can evaporate inside the heat pipe evaporators. Following the subsequent condensation, heat is released again, and the adsorbents get regenerated. By using a small boiler evaporator, two cylinders inside the adsorbers, and two flexible pipes attached to a vapor chamber and a pipe to permit vapor flow, it is possible to put together a siphon that will get the heat out of the system (Wang and Oliveira). It is also necessary to place the heat pipe condenser on the exterior of the refrigerant evaporator and connect that evaporator with a loop heat pipe. Using the two arcs of the heat pipes as an extra circuit for the ammonia and connecting them to the first ammonia circuit, the heat inside the evaporators goes beneath that inside the refrigerator itself. The ammonia will evaporate and the condense on the evaporator’s exterior – thus moving the heat via natural convection. This would produce at least 300 W of cooling power (Wang and Oliveira).
The system patented by Xia et. a. and referenced earlier utilized heat pipes with a chiller made of water and silica gel. The heat pipes increase the transfer flux and makes the manufacturing costs go down, because the pipes are cheaper to manufacture than the dual water evaporator/condenser, which in Xia’s system is replaced by one evaporator that uses methanol. Using copper tubes in the water evaporator will allow the pores in the metal to increase the evaporation efficiency, which allows for a smaller evaporator. The methanol moves from the outside of the tubes to the inside through evaporation and condensation, and the system gathers the condensate. This allows for optimal efficiency when water enters the system at 85 degrees Celsius. See Table 1 to note how different temperatures affect the cycle time and overall power:
| Hot water temp. (Celsius) | Cooling water temp. (Celsius) | Chilled water temp. (C) – inlet | Chilled water temp. (C) – outlet | Cooling power (kW) |
| 78.8 | 31.3 | 20.5 | 16 | 8.32 |
| 81.8 | 31.3 | 20.7 | 16.3 | 9.33 |
| 86.8 | 30.9 | 21.1 | 16.3 | 10.62 |
| 59.7 | 30.4 | 20.5 | 18.2 | 4.8 |
| 69.1 | 30.3 | 19.6 | 16.2 | 7.57 |
| 84.4 | 30.5 | 21.5 | 16.5 | 10.88 |
| 85.3 | 30.6 | 20.9 | 16.1 | 10.44 |
| 80.3 | 30.2 | 15.8 | 12.1 | 8.26 |
| 82.5 | 30.4 | 15.8 | 11.9 | 8.69 |
| 83.8 | 30.8 | 15.4 | 11.8 | 8.6 |
| 81.8 | 31.3 | 20.7 | 16.3 | 9.33 |
Table 1 – Wang and Oliveira
Heat pipes will help reduce the cost of adsorption cooling systems. However, other issues regarding the appropriateness for larger applications also include their size. Designers must come up with ways to maximize the external and internal heat transfer, increase SCP and COP, and improve heat management overall.
To enhance external heat transfer, the heat exchange area must increase. This might seem ironic, given the need to reduce the size of the system, but extended the surface where heat exchange can happen is possible in a number of ways. Manufacturers can make tubes with fins, plate-fin heat exchangers and simple plate heat exchangers too. This will work even more efficiently if the system uses a sorbent does not shrink or swell significantly. This does raise the adsorber’s thermal capacity, which means that the system will need to be effective in its heat management for this to work. Also, the system will need to maintain a consistently high pressure level to work.
Another solution is to use adsorbents that are consolidated or composite. Particularly consolidated adsorbents that can conduct heat at a high level can lead to higher heat transfer. Zeolite and expanded graphite have been used to make consolidated composite compounds that produced SCP results that were more than four times higher than just using zeolite alone. Making a compound from metallic foam and zeolite resulted in conductivity more than 22 times greater than zeolite alone. Using a combination of organic binder and activated carbon produced an SCP 90 percent higher than with just using powdered carbon. Combining calcium chloride and activated carbon created a cooling density a full third higher than what resulted with powder calcium chloride (Critoph; Wang et. al.)
Other combinations in formulating adsorbents also show significant promise. Silica gel and expanded graphite produce thermal conductivity as high as 60 times that of granular silica gel alone. Combining expanded graphite and manganese chloride showed almost no limits in potential heat transfer. The risk, though, is that because consolidated absorbents do not transfer mass as well as granular ones, adsorption can plummet when refrigerants such as methanol and water evaporate at standard atmospheric pressure (Wang and Oliveira). This means that testers must also measure the permeability of compounds in addition to heat transfer coefficient, because of the risk of losing adsorption with high permeability.
Coating adsorbers is another way to improve performance, particularly in situations where high SCP is more important than high COP. This process works through the spike in wall heat transfer coefficient that happens when the friction between the adsorbent and the heat exchange surface goes down. Using zeolite crystal monolayers on tube metal surfaces, researchers achieved a heating rate in excess of 1500 W per sorbent kilogram. Sliding the adsorbent bed into a larger graphite plate reduces the contact between the adsorbent and the the transfer fluid, but the ratio of adsorbent mass to inert material goes up, because the adsorbent bed can thicken more. However, this ratio ruins the COP, which means that engineers really have to nail down effective heat management. Using a system with coated tubes generally provides about 3 times as much cooling power than a system with only zeolite pellets. In the right application, coating the tubes makes a lot of sense.
To increase COP, engineers will want to work on advanced cycles and enhanced heat management. When adsorption coolers work with heat recovery, one adsorber sends heat to a colder one – one that is just at the beginning of the generation phase. This could go on until both adsorbers have temperatures that are about the same, but this will generally wane when the difference between the two gets between 5 and 15 degrees Celsius. At this point, both adsorbers get hooked up to a heat source and a heat sink, to bring generation and adsorption to their completion (Wang and Oliveira). This allows about 35 percent of the energy that had been sent to each adsorber to be recovered. Cycles that use thermal wave will normally result in higher COP figures. At the beginning, the temperature of the inlet fluid will be the same as the heat source and heat sink, but the outlet fluid temperatures will vary over time.
In the adsorption process, when heat regenerates the adsorbent, the fluid carrying the heat runs through the cooling adsorber, the source of the heat, the warming adsorber, and then the heat sink. Even if the most sophisticated equipment involved is a reversible pump, it is possible to recover about two thirds of the energy that each adsorber receives. By using a two bed adsorption air conditioner that uses zeolite and water as the working pair, it is possible to see COP values between 1 and 1.6, depending on the particular ambient temperature. With a pairing of ammonia and activated carbon, the COP will vary between 0.42 and 1.19, again depending on the temperaure (Critoph).
An area in which engineers can improve adsorption cooling system performance is by preventing the production of hydrogen gas. Using aluminum pipes with no coating and exposing them to water vapor inside a vacuum can lead to the formation of hydrogen gas and aluminum hydroxide radicals (Yong and Wang). The problem that this can cause is a loss of vacuum, that can undermine the effectiveness of the cooling process. One possible solution is to use stainless steel instead of aluminum, but stainless steel has significantly less conductivity, which means higher resistance to heat transfer. One system, patented by Nagashima, uses an aluminum casing for the heat exchanger, but uses a stainless steel case for the adsorber. This system uses two heat exchangers – one for transferring heat between the transfer fluid and the refrigerant, and another with the adsorbent. The stainless steel case for the adsorber will develop a silicon oxide film that will keep hydrogen gas from forming. Another solution is to bond the adsorbent matrix right to the plates; this keeps aluminum corrosion from becoming a possibility (Yong and Wang).
These objections being said, there are several reasons why finding ways to use solar energy with adsorption cooling systems is still an important area of research, for further study. After all, the months in which cooling and refrigeration are needed the most are also the months that offer the greatest exposure to solar energy. In addition to cooling air, other machines have been devised that offer a number of cooling operations, including the one patented by Wang et. al. that will produce ice and hot water. This machine puts the adsorption cylinder in the top water tank. When the sun is up, the energy heats the water in the tank – and the adsorption cylinder inside the cooling unit (Wang et. al.). At night, the water cools – and so does the adsorption cylinder.
At the present time, there is considerable research going on in making solar energy work for adsorption cooling. Throughout the world, there are currently 115 patents pending in this field, 80 percent of which are from the United States, Japan and China. Since 2000, the number of new patents filed has increased year – by as much as 110 percent (Yong and Wang). Clearly, this is an area that will continue to see growth as more of the hurdles to efficient adsorption cooling processes are eliminated.
In addition to those areas already mentioned, further study is still needed in several areas relating to solar energy and adsorption cooling. Heat pipe technology does remain a promising option, depending on the material of the pipes. Because heat transfer will increase and the pipes will make for a simpler structure, research in ways to make these pipes cheaper and enhance reliability is needed. Also, given the increased use of computers to control the functioning of cooling systems, ways to interface computers with the work of an adsorption system would be another promising goal of research. Other areas of research include finding ways to boost the recovery of thermal energy while keeping the system reasonably simple, as well as improving the recovery rate of refrigerant mass. Finding ways to improve the adsorbent bed regeneration process is also a way to make the system work more efficiently. Carrying out research in applied materials will find even better coatings and working pairs, so that the process happens more efficiently.
Finally, finding ways to make the collection of solar energy in photovoltaic cells more efficient is of paramount importance. While these metrics have improved over time, the fact remains that a significantly high amount of solar energy simply gets wasted in the collection process. Advances in this area will benefit all applications of solar energy, not just adsorption cooling or other heat-related uses of the sun’s energy. All of the advances of the past four decades notwithstanding, there is plenty of room for the next round of innovations that will liberate the planet from the use of fossil fuels.
Conclusions
Solar energy, while expensive to install, does have a number of positive applications that could end up eliminating much of the air pollution that comes from the generation of power. While more traditional absorptive cooling systems require electricity (and the burning of fossil fuels through the consumption of coal), solar energy collection systems can not only provide the electricity that a house needs, it can also collect the heat needed to power an adsorption cooling system, and send that heat throughout the system. Many advances have been made in just about every field of alternative energy since the seemingly infinite flow of fossil fuels was threatened in the 1970s. While the trend in the United States has demonstrated a perpetual tendency to think in the short term, when it comes to public policy, the fact that there are more patents pending in this area in the United States than in any other country is a source of encouragement. One hopes that current awareness of the growing demand for fossil fuels will encourage continued research and discovery in alternative energy. The population and prosperity explosion in south Asia, for example, means that even if every person in the United States traded in their traditional gasoline-powered car for a hybrid, the demand from…